Variation of solvent density and low-resolution ab initio
phasing
Andrei Fokine
LCM3B, UMR 7036 CNRS, Faculté des Sciences, Université Henry Poincaré,
Nancy I, 54506 Vandoeuvre-lés-Nancy, France
e-mail: fokine@lcm3b.uhp-nancy.fr
Abstract
The bulk solvent plays a key role in the direct
phasing at low resolution. The connectivity-based phasing method has been
tested for the crystal of the tRNAAsp-aspartyl-tRNA
synthetase complex. The neutron diffraction data have been measured for
different solvent contrast masking different components of the crystal. The
obtained low resolution images are perfectly correlated with the solvent level.
1. Introduction
Low-resolution Fourier syntheses provide a very useful information about molecular packing and the shape of molecules. Such low-resolution images can be obtained by direct phasing of a single set of experimental modules (Lunin et al., 2000a). The information supplied by ab initio phasing methods is inestimable when conventional phasing techniques do not work. The low resolution phases can be used as a starting point for phase extension. Additionally, the knowledge of molecular position can facilitate the solution of molecular replacement problem. Here the ab initio phasing method based on topological properties of density maps is considered. This method employs the expected connectivity of a Fourier synthesis as an additional information to obtain phases (Lunin et al., 2000b). Among the known low-resolution direct phasing techniques this method is considered to be the most promising. There are several examples when this method allowed to determine molecular positions, molecular shapes (Lunin et al., 2000a; Lunin et al., 2000b) and even secondary structure elements (Lunin et al., 2002).
Macromolecular crystals contain a large part of the bulk solvent
whose contribution to low-resolution reflections is very important. At low resolution the assumption
of a uniform scattering density distribution in the solvent region is held well
and the value of solvent scattering density can be estimated from the
composition of crystallization solution. In X-ray structural analysis the
electron density of the bulk solvent varies in the small range 0.33 – 0.41 e/Ĺ3
(Kostreva, 1997). The solvent electron density is close to the average electron
density of a typical protein 0.43 e/Ĺ3 (Kostreva, 1997; Carter et
al., 1990) and less than that of DNA / RNA. In neutron diffraction
experiment, the solvent scattering density can be varied in a large range by
changing the ratio of D2O / H2O in the mother liquor. The
solvent scattering density can be made lower equal or greater than the average
scattering density of a protein or DNA / RNA. Such possibility
of choice of an appropriate solvent scattering density is the basis of the method of contrast variation (Jacrot, 1976).
The connectivity properties of
low-resolution Fourier syntheses essentially depend on the solvent
contribution. Therefore, the results of direct phasing which uses these properties
as the selection criterion depend on the solvent scattering density. The
purpose of the current work was to study the role of
the bulk solvent in the connectivity-based direct phasing.
In this paper we present the results of ab initio phasing of three sets of neutron diffraction data from the same crystal of the tRNAAsp-aspartyl-tRNA synthetase complex (Moras et al., 1983) measured for different solvent contrast masking different components of the crystal. The obtained low resolution images are perfectly correlated with the solvent scattering density level.
2. Phasing method
The
basic idea of the connectivity based phasing method (Lunin et al.,
2000b) consists in the observation that topological properties of high density
regions of the Fourier syntheses are different for properly phased syntheses
and for those calculated with random phases.
Let ρ(r) be a Fourier
syntheses calculated on a finite grid and N be a number of grid points in the
unit cell. The high density region Ω(α), corresponding to the
relative volume α is defined as a set of αN grid points of highest
density. For a correctly phased low-resolution synthesis this region would be
composed of a small number of isolated ‘blobs’ corresponding to independent
molecules if the cut-off level α is chosen appropriately. The number of
these blobs is usually equal to the number of molecules in the unit cell. The
blob volumes (measured in the number of grid points) must be equal between
themselves if all molecules are linked by crystallographic symmetries and must
be approximately equal if non-crystallographic symmetry is present. On the other
hand, randomly phased syntheses are likely to show infinite merged regions or a
large number of ‘drops’.
The phasing procedure consists in following
steps.
1)
A
large number of random phase sets are generated. The phases are generated with
the uniform distribution at the beginning of the procedure or in accordance
with a known phase distribution if this information is already available.
2) For every generated phase set the Fourier synthesis is calculated using the experimental structure factor modules.
3) The high density regions of each calculated synthesis are subjected to connectivity analysis in order to determine the number of separated connected components in the unit cell and to calculate their volumes.
4)
If
high density regions consist of a desired number of components, the
corresponding phase set is considered as admissible and is stored, otherwise
the phase set is rejected.
5)
After
a reasonable number (about one hundred) of admissible phase sets have been
selected, they are averaged in order to produce the corresponding ‘best’ phases
![]() and figures of merit
and figures of merit ![]() , which reflect the spread of the admissible phase sets
, which reflect the spread of the admissible phase sets
![]() . (1)
. (1)
Here M is the number of selected phase sets and ![]() is the value of phase
of the structure factor with the index h
in the j-th selected phase set. It
should be noted that the optimal alignments of the phase sets in accordance
with the permitted origin shifts (Lunin & Lunina, 1996) must be performed
before averaging.
is the value of phase
of the structure factor with the index h
in the j-th selected phase set. It
should be noted that the optimal alignments of the phase sets in accordance
with the permitted origin shifts (Lunin & Lunina, 1996) must be performed
before averaging.
The phasing method is described in more details in Lunin et al. (2000b).
3. Test object
Cubic form of tRNAAsp-aspartyl-tRNA synthetase
complex
The crystals of the cubic form of
the tRNAAsp-aspartyl-tRNA synthetase complex
belong to the space group I432 (48 asymmetric unit / unit cell) with a unit cell parameter of 354 Ĺ.
The asymmetric unit contains one protein homodimer and two molecules of tRNA. The crystals contain 82% of the bulk solvent. The
protein and tRNA molecules occupy 14 and 4 % of the unit cell
respectively. The structure was solved by molecular
replacement (Urzhumtsev et al., 1994) using 15-8 Ĺ resolution X-ray diffraction data and high
resolution (3
Ĺ) model of the complex obtained from the orthorhombic crystal (Ruff et al.,
1991).
Neutron diffraction data
Three sets of neutron diffraction data, all complete at low resolution (from infinity to 24 Ĺ), were measured for the same crystal of the complex with different concentrations of D2O in the mother liquor (Moras et al., 1983).
The first data set was measured without D2O in the mother liquor which corresponds to a very low solvent scattering density. This data set corresponds to the full complex molecule.
For the second data set the concentration of D2O was chosen so that the solvent scattering density was equal to the average density of the protein. Thus the protein was masked by the solvent and only tRNA molecules gave a signal in diffraction.
The third data set was measured with the solvent scattering density matching the average density of tRNA, therefore this data set corresponds to the protein molecules only.
These three data sets were used for the direct
phasing.
4. Ab initio phasing of experimental neutron data sets
It should be noted that for the direct
phasing described below we used only a general information (known a priory)
such as the number of molecules in the unit cell and the relative unit cell
volumes occupied by molecules.
Phasing of the data set
corresponding to the full complex molecules
The crystals contain one densely
packed dimer of tRNAAsp-aspartyl-tRNA
synthetase complex in
the asymmetric unit. Since the space group I432 has 48
symmetry operations, it is natural to expect that a low-resolution
synthesis with correct phases would show 48 blobs of equal volumes
corresponding to the complex molecules.
Reflections in the resolution range ∞-45 Ĺ were used (37 reflections) for phasing and phases were
generated with the uniform distribution. The selection criterion was formulated
as follows: the high density region occupying 5% of the unit cell (α =
0.05) must be composed of 48 connected blobs of equal volumes. 100 selected
variants were stored after about 89000 generations. The selected variants were averaged
to produce the ‘best’ phases ![]() and the figures of merit
and the figures of merit ![]() . The ab initio phased synthesis calculated at 45 Ĺ is shown in
Fig. 1. From this synthesis, the position of the complex molecules in the unit
cell can be determined.
. The ab initio phased synthesis calculated at 45 Ĺ is shown in
Fig. 1. From this synthesis, the position of the complex molecules in the unit
cell can be determined.
Phasing of the data set
corresponding to tRNA molecules
The asymmetric unit contains 2 tRNA
molecules related by non-crystallographic symmetry and separated at the surface
of the complex. It is natural to expect that the low-resolution synthesis with
correct phases would show 48 blobs of equal volumes corresponding to the first
tRNA molecule and 48 blobs of equal volumes corresponding to the second tRNA
molecule. The volumes of blobs corresponding to tRNA molecules related by
non-crystallographic symmetry must be approximately equal.
As previously, reflections in the
resolution range ∞-45 Ĺ were used for phasing. Phases were generated with the uniform
distribution. The selection criterion was formulated as follows: the high
density region occupying 3% of the unit cell (α = 0.03) must be composed
of 48 connected regions of equal volumes corresponding to the first tRNA
molecule and 48 connected regions of equal volumes corresponding to the second
tRNA molecule; the ratio of volumes of the connected regions corresponding to
different tRNA molecules must be higher than 0.7. It was a priory known
that the tRNA molecules occupy 4% of the unit cell therefore the slightly
higher cut-off level of 3% was used. About 270000 random phase sets were
generated, 100 sets from them satisfied the selection criterion. The
corresponding ab initio phased synthesis is shown in Fig. 2. From this
synthesis the positions of the tRNA molecules in the unit cell can be
determined unambiguously.
Phasing of the data set
corresponding to the protein molecules
Similarly to two previous cases, reflections in the resolution range ∞-45 Ĺ were used and phases were generated with the uniform distribution. The selection criterion was formulated as follows: the high density region occupying 5% of the unit cell (α = 0.05) must be composed of 48 isolated blobs of equal volumes. 100 selected variants were stored after about 50000 generations. The ab initio phased synthesis (Fig. 3) shows clearly the position of the protein homodimer.
5. Conclusion
The current study shows the robustness of the connectivity-based
phasing method which was capable to determine unambiguously the position of
both tRNA molecules and that of protein homodimer in the tRNAAsp-aspartyl-tRNA
synthetase complex.
The results confirm that the bulk solvent plays the key role in the connectivity-based direct phasing. The phasing of three data set measured from the same crystal led to completely different images depending on the solvent scattering density.
The author thanks A. Urzhumtsev, V. Lunin, N. Lunina, E. Chabriere and P. Afonine for useful discussions.
The work was done in the frame of the pole “Intellegence Logiciels” CPER-Lorraine and in collaboration with CCH, Nancy, and was supported by the grant of “Region Lorraine”. The author is a member of GdR 2417 CNRS.
References
Carter C.W., Jr., Crumbley, K.V., Coleman, D.E.,
Hage, F. & Bricogne, G. (1990). Acta Cryst. A46, 57-68.
Jacrot, B.
(1976). Rep. Prog. Phys. 39, 911
Kostrewa, D.
(1997). CCP4 Newslett. 34, 9-22.
Lunin, V.Y. & Lunina, N.L. (1996). Acta Cryst., A52,
365-368.
Lunin, V.Y., Lunina, N.L., Petrova, T.E., Skovoroda, T.P., Urzhumtsev, A.G. & Podjarny A.D. (2000a). Acta Cryst. D56, 1223-1232.
Lunin, V.Y., Lunina, N.L., Urzhumtsev, A.G. (2000b). Acta Cryst., A56, 375-382.
Lunin, V., Lunina, N., Podjarny, A., Bockmayr,
Urzhumtsev, A. (2002) Z. Kristall., in press.
Moras,
D., Lorber. B., Romby, P., Ebel. J.P., Giege, R., Lewit-Bentley, A., Roth, M.J. (1983) Biomol. Struct. Dyn., 1,
209-223
Ruff, M., Krishnaswamy, S., Boeglin, M., Poterszman,
A., Mitschler, A., Podjarny, A., Rees, B., Thierry J.-C. & Moras D. (1991) Science,
252, 1682-1689.
Urzhumtsev, A.G., Podjarny, A.D. & Navaza, J. (1994). CCP4 Newslett. 30, 29-36.
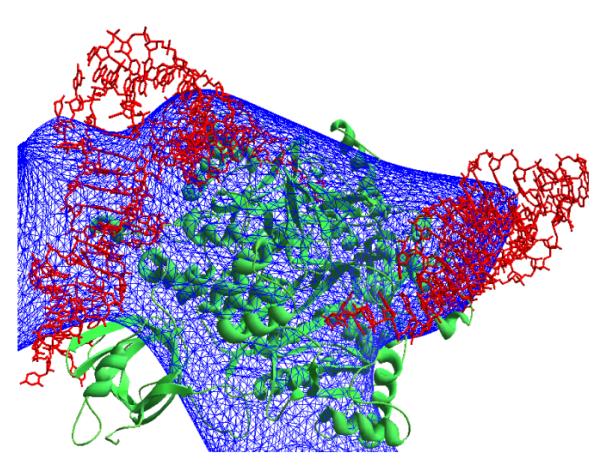
Fig. 1 Synthesis calculated at 45 Ĺ resolution
(37 reflections) using experimental modules corresponding to the full complex
molecule and phases obtained ab initio.
Protein
molecule is shown in green and tRNA molecules are shown in red.
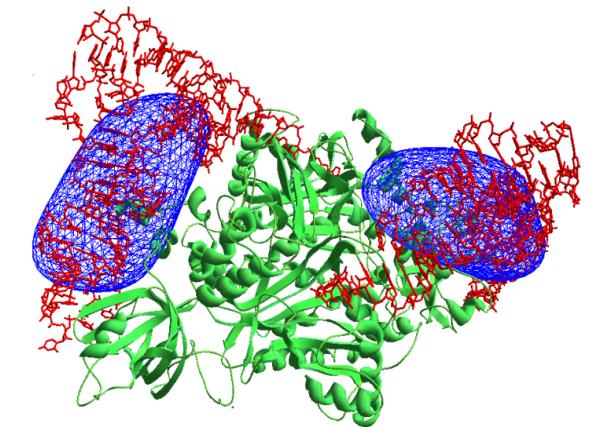
Fig. 2 Synthesis calculated at 45 Ĺ resolution
(37 reflections) using experimental modules corresponding to the tRNA molecules
and phases obtained ab initio.
Protein
molecule is shown in green and tRNA molecules are shown in red.
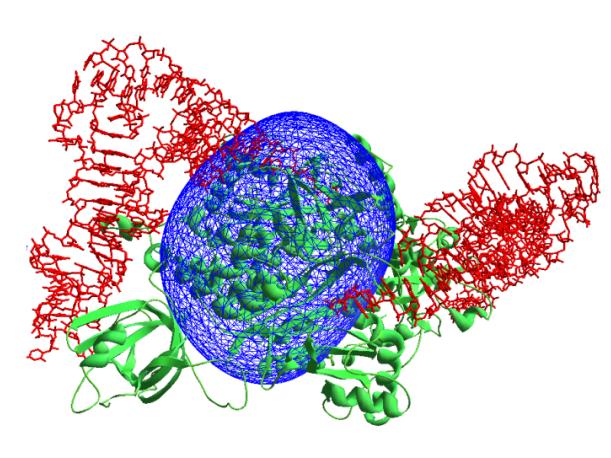
Fig. 3 Synthesis calculated at 45 Ĺ resolution
(37 reflections) using experimental modules corresponding to the protein
molecules and phases obtained ab initio.
Protein
molecule is shown in green and tRNA molecules are shown in red.