Modelling of bond electron density by Gaussian scatters at subatomic resolution
P. Afonine 1,2, V. Pichon-Pesme1, N. Muzet1, C. Jelsch1, C. Lecomte1 & A. Urzhumtsev1
1 LCM3B, UPRESA 7036 CNRS, Faculté des Sciences, Université Henri Poincaré, Nancy I, 54506, Vandoeuvre-lès-Nancy, France
2Centre Charles Hermite, LORIA, Villers-lès-Nancy, 54602 France
Abstract
At resolutions of 1-2 Å and lower, traditional for macromolecular crystallography, the electron density of the crystal unit cell is modelled by a sum of contributions from individual atoms (plus the bulk solvent). The contribution of each atom is calculated as a spherical or an elliptic function centred at the atomic position. Such models are not adequate at a subatomic resolution, higher than 1 Å, as proven by residual Fourier maps. In particular, these maps show extra density peaks between the bonded atoms. A modelling of this density by placing extra ‘gaussian’ electrons at the corresponding positions allows the improvement of crystallographic criteria. This new model allows also the determination of some important characteristics of the crystal such as critical points of the electron density distribution.
1. Introduction
Crystal models play several roles. First, they give a possibility to describe the content of a crystal by a finite number of parameters, usually with a clear physical meaning. Secondly, these models allow the determination of properties which are not available directly from the experiment. For example, model parameters can be used to calculate the electron density distribution while the diffraction experiment gives only a limited set of its Fourier coefficients and allows thus the calculation of Fourier syntheses only at a limited resolution. Naturally, such a model should be quite precise and detailed.
In macromolecular crystallography, for many years the usual resolution of the experimental data was about 2-3 Å. At such a resolution, the models, composed from isotropic atoms and refined using crystallographic restraints or constraints, give a good description of experimental diffraction data. During last years, many macromolecular structures were reported at atomic resolution, about 1 Å (for a review, see Dauter et al., 1997; Longhi et al., 1998). Such data require more detailed models. In particular, anisotropic thermal displacement parameters become necessary. An introduction of extra parameters is justified by increased number of structure factor magnitudes as confirmed by R-free (Brünger, 1992) calculations. Recently, several cases were reported where macromolecular crystals diffracted to the resolution higher than 0.9 Å. An example is aldose reductase (Lamour et al., 1999) crystals of which diffract to 0.64 Å even when the size of this protein is quite large, 315 amino acid residues. At such a subatomic resolution, the spherical electron density model even with anisotropic thermal parameters is already inadequate. In particular, difference maps show significant density peaks around the atoms, essentially at the interatomic bonds (Housset et al., 1994; Lamzin et al., 1999). On the other hand, a large amount of data could allow the detailed study of physical properties of electron density of the crystal.
In small molecules crystallography such subatomic resolution is usual and the problem was solved by introduction of multipolar models (Stewart, 1969; Hansen & Coppens, 1978). Here the density of each atom is modelled by a function:
where the total atomic electron density (ratom) is decomposed into three terms corresponding to the core electrons, valence electrons and non-spherical part of the valence electron distribution as a multipole density (see Hansen & Coppens, 1978, for details and notation). The number of parameters for each atom grows with the number of included spherical harmonics. For traditional models, a multipolar atom, different from a hydrogen, can have up to 28 parameters (18 multipolar, 3 positional, 6 temperature parameters, 1 occupancy). Recently, Pichon-Pesme et al. (1995) and Jelsch et al. (1998) demonstrated the transferability of such models to macromolecular crystals when a large enough number of structure factor magnitudes allows this (in other words, when the resolution of the diffraction data set is high enough). Such models have been successfully refined for crystals of crambin (Jelsch et al., 1999), for a scorpion toxin (Housset et al., 2000) and for an aldose reductase complex (Guillot et al., 2000), respective resolution of these crystals is 0.54, 0.9, and 0.65 Å.
Some other protein crystals, for example those of other complexes of aldose reductase, diffract to slightly lower resolution, about 0.9 Å. Use of multipolar atoms and refinement of hydrogen atoms reduces the ratio Ndata/Nparameters to the limits when such type of a model can be hardly used. The major risk is, as in any modelling, to overfit the data introducing too many parameters in the model.
In order to answer this problem, a project was started to develop a simplified molecular model which is more detailed than a usual model of anisotropic atoms but contains less parameters than a multipolar model. This model can be used when the amount of experimental data is not sufficient enough to use multipolar models. Additionally, such a model would accelerate the calculations of crystallographic values (structure factors, electron density, and electrostatic potential) which are quite time consuming when multipolar models are employed for macromolecules.
2. Model of bond electrons
As traditionally for macromolecular crystals, incorrect parts of an available model are highlighted in difference density maps. In particular, so called ‘deformation density maps’ can be calculated as the Fourier series
Here V – unit cell volume, k – scale factor, Fobs(h) – observed structure factor amplitudes, jbest(h) – best phases available, Fsph(h) and jsph(h) – structure factors amplitudes and phases obtained from spherical atomic model. These maps show an excess and a missed part in the available model by negative and positive peaks whose size does not allow seeing them at lower resolution. Such maps show the redistribution of the electron density due to formation of interatomic bonds and other interactions. Among various ‘deformations of density’, large peaks are seen most clearly on the bonds (Fig. 1A). This extra density, which represents reorganisation of electrons to bonding, seems to be the major need for a necessary model modification.
Previously, several attempts have been done (Ewald & Höln, 1936a, 1936b); Brill, 1960; Hellner, 1977; Dietrich & Scheringer, 1978; Scheringer, 1980; Pietsch, 1981; Pietsch & Unger, 1981; Pietsch, 1985; Pietsch et al, 1986) to model this density by placing there an additional scattering matter, a kind of pseudo atom. All these attempts were done with small-molecular crystals with a small number of diffraction intensities. On contrary, macromolecular crystals have a very large number of reflections at such a subatomic resolution. This allows an easy application of the R-free methodology (Brünger, 1992) which has been shown as a powerful tool to indicate the data overfitting. First checks (Cetin et al., 2000) showed a feasibility of this modelling.
3. Preparation of the test model
More detailed tests were done using the leu-enkephalin peptide (Wiest et al., 1994). This pentapeptide (Tyr1-Gly2-Gly3-Phe4-Leu5) crystallises in space group P212121 with unit cell parameters a = 10.851 Å, b = 13.095 Å, c = 21.192 Å and Z = 4. The diffraction data were collected to 0.43 Å. However, the tests described below were done at a lower resolution, d > 0.56 Å (sinq/l < 0.89 Å-1), beyond which the completeness of data is insufficient (Sheldrick, 1990), see Fig. 2. At the resolution of 0.56 Å the number of independent reflections is 8707 and completeness for the last high-resolution shell is higher than 60 %. The enkephalin model contains 43 non-hydrogen and 43 hydrogen atoms. A refined multipolar model (M1) was obtained (Wiest et al., 1994) using the program MOLLY (Hansen & Coppens, 1978). This refinement was done without separation of the data set into work and test (R-free) subsets.
Because the R-free values were important for our tests (one of the indicators of modelling progress) and in order to estimate the quality of previously refined model (M1) in terms of ‘R-free’, a usual procedure was applied (Brünger, 1993). First, a test set was chosen, composing 20% of the total amount of reflections belonging to 0.56-11.0 Å resolution. This selection was done randomly and uniformly in several resolution shells. Random and independent errors were introduced into atomic coordinates of M1 model by performing molecular dynamics simulation at 2000K with subsequent energy minimisation using CNS (Brünger at al, 1998). Such procedure removed some of the ‘memory’ of previous refinement towards test set and gave the model differing from M1 by ~0.06 Å shift in coordinates. Then, this model was refined using only the 80% rest of reflections (work set) by the program MOPRO (Guillot et al., 2001) at the exactly same conditions as previously, reported by Wiest et al (1994). The desired R / Rfree-factor statistics for the model obtained (M2) is summarised in Table 1.
4. Test of bond electron models
All the subsequent tests were done with the program suite SHELX (Sheldrick & Schneider, 1997). No sterochemical constraints were used in these tests.
First of all, the conventional anisotropic refinement of enkephalin was done at 0.56-11.0 Å resolution in order to have reference values for such type of models at this resolution (model A1 in Table 2). It is not surprising that the results of standard anisotropic refinement (R = 9.08 %, Rfree = 9.74 %) are worse than those after multipolar refinement (R = 7.90 %, Rfree = 8.63 %; M2 model in Table 1). This tendency is true in every resolution shell (Fig. 3).
For the first test with bond electron (BE in what follows) models, standard hydrogen atoms were used. These atoms were placed in the middle of interatomic bonds (as in Fig. 1B). The optimal starting values of occupancies for BEs were found as 0.5, agreeing with previous tests (Cetin et al., 2000) and the starting values of isotropic temperature factors were taken equal to the average temperature parameters of the neighbouring atoms. Different set of parameters were refined and various refinement strategies were tested. In many cases, including the case of anisotropic BEs, the R-free criterion increased or refinement was unstable (details of these numerous tests will be discussed elsewhere). The best improvement in both R and R-free values, to 8.43 and 9.16 % (model BE1 in Table 2), was obtained when refining the following parameters:
- for each non-hydrogen atom of the model: coordinates, anisotropic temperature factor and occupancy;
- for each hydrogen atom of the model placed according to ideal stereochemistry: isotropic temperature factor (while occupancy is fixed equal to 1);
- for each bond electron (BE): isotropic temperature factor and occupancy.
It is important to note that this refinement decreased the occupancies of the enkephalin atoms showing the redistribution of the density to newly placed BEs.
The number of BEs is approximately equal to the number of bonds (some extra BEs can be also introduced for lone pair electrons) or, in other words, is approximately equal to the total number of atoms in the molecule. Therefore, the number of additional parameters in BE-model can be estimated as 2 parameters per atom, resulting in 12 parameters per atom in total.
The model was improved when the BEs, taken previously as hydrogens, were replaced by Gaussian scatterers defined from DFT calculations by program SIESTA (Sanchez-Portal et al., 1997) as follows. First of all, the theoretical deformation density maps were obtained for all types of residues as the difference between the exact electron density distribution calculated by quantum-chemical methods and that calculated from the model using standard scattering factors for neutral atoms. For these calculations, each idealised single residue was taken as an isolated molecule in gas phase. The peaks at the bonds were approximated by a single-gaussian function, and the corresponding distance between the peak centre and the neighbouring atoms was calculated. Then, such gaussian peaks were placed in the covalent bond positions of enkephalin. Similarly to the previous case, the starting values of isotropic temperature factors were taken equal to the average temperature parameters of the neighbouring atoms. The refinement of this model decreased the R and R-free factors to 8.12 and 8.76 % (model BE2 in Table 2). The behaviour of R-factors as a function of resolution is shown in Fig. 3.
Summarising, it should be noted that the proposed BE-model:
- is better then the best anisotropic model;
- requires about 12 parameters per atom, in comparison with 10 parameters for the anisotropic model and 28 for a multipolar model;
- gives values of overall R- and Rfree-factors close to that for the refined multipolar model.
5. Further validation of bond electrons models
A lower value of the R-free factor is a good proof of a higher quality of BE-models in comparison with classical anisotropic models. However, more tests were done to confirm the quality and physical meaning of the BE-model.
First, the rigid-bond test (Hirshfeld, 1976) showed that BEs do not cause unphysical perturbations in the thermal parameters of atoms. A refinement even without rigid-bond constraints gave the rigid-bond criterion at the same level as for the multipolar model (Fig. 4). An inclusion of the rigid-bond criterion into refinement (not shown) improved the similarity of the projections of thermal ellipsoids on the bond without increasing the R and R-free values.
The second check consisted in verification of a predicting power of such BE-models. Multipolar models allow the calculation of accurate electron density. In small-molecule crystallography, these electron density maps are called ‘experimental’ because the models used for their generation are obtained from the experimental data. One of the important features of this distribution is critical points, where the gradient of the density is equal to zero (Bader, 1990). In particular, these points allow the characterisation of atomic interactions. Each such point is characterised by three numbers which are the eigenvalues of the normal matrix of the electron density calculated at this critical point (the matrix of second derivatives with respect to three coordinates). These values allow one to characterise the type of covalent bonds. A special role is played by density Laplacian which is the sum of the three eigenvalues.
In order to obtain the parameters of critical points for BE-models, a special program was written allowing a very fast calculation of the electron density map, exact maps of the gradient of electron density and that of the density Laplacian directly from the BE model. Electron density maps calculated from the best available isotropic and anisotropic models did not reproduce the critical points derived from the multipolar model. On the contrary, the BE2 model gave both the correct position of the critical points and, for most of bonds, the eigenvalues were very close to those obtained from the multipolar model (Table 3). A few cases with incorrect eigenvalues, for example that for the C=O bonds, prove that for some types of bonds the parameters of BEs, including their position, should be improved in further studies.
6. Conclusions and discussions
Several independent criteria confirmed that modelling of the density on the interatomic bonds plays the major role in the improvement of anisotropic models on the way to multipolar models. The simplest way to improve an anisotropic model is its completion by appropriate gaussian scatters for bond electrons. The available software does not allow the complete refinement of parameters of these electrons as it seems to be necessary. Nevertheless, by refining only the shape and not the position of these BEs, R and R-free factors can be significantly decreased in comparison with anisotropic models. Moreover, our tests demonstrated that the refinement of BE-models gives the values of R- and Rfree-factors similar to those for multipolar models.
These models reproduce the experimental structure factor values better thus allowing the calculation of improved Fourier syntheses using the experimental structure factors. In addition, such models provide electron density maps that cannot be obtained using isotropic or anisotropic models. The maps reproduce important features of the electron density distribution, for example, its critical points.
A slight decreasing in occupancies of C, N and O atoms in the refined BE-model indicates the tendency to conserve the total charge of the model without electroneutrality constraint; this confirms physical meaning of such a modelling.
The BE-models are described by a smaller number of parameters than multipolar models (approximately 2 times less) and therefore can be used at lower resolution when the number of experimental structure factor magnitudes is smaller. In addition, gaussian scattering factors of all atoms of the model (a 5-gaussian approximation of atomic scattering factor works well at least up to the resolution of 0.25 Å, see International Crystallographic Tables, 1998) should allow a very fast and direct calculation of the exact maps of the gradient of electron density and the maps of the density Laplacian.
The refinement of BE-models does not require any special refinement programs. Nevertheless, more sophisticated software would allow the use of special criteria and refinement strategies.
The work is in progress.
Acknowledgment
The authors thank C. Katan, M. Souhassou, N.-E. Ghermani for useful discussions of high-resolution features of crystals, I. Uson and G. Sheldrick for their help with the use of SHELX program suite and V. Lunin for fruitful discussions. The authors are participants of the GdR 2417 CNRS.
References
Bader, R. W. F. (1990). Atoms in Molecules. A Quantum Theory. Oxford University Press.
Brill, R. (1960). Acta Cryst., 13, 275-276
Brünger, A. T. (1992). Nature, 355, 472-475
Brünger, A. T. (1993). Acta Cryst., D49, 24-36
Brünger, A. T., Adams, P. D., Clore, G. M., DeLano, W. L., Gros, P., Grosse-Kunstleve, R. W., Jiang, J.-S., Kuszewski, J., Nilges, M., Pannu, N. S., Read, R. J., Rice, L. M., Simonson, T. & Warren, G. L. (1998). Acta Cryst., D54, 905-921.
Cetin, A., Pichon-Pesme, V., & Urzhumtsev, A. (2000). University of H. Poincaré, Nancy 1, LCM3B; internal report.
Dauter, Z., Lamzin, V. S. & Wilson, K.S. (1997). Curr. Opin. Struct. Biol., 7, 681-688
Dietrich, H. & Scheringer, C. (1978). Acta Cryst., B34, 54-63
Ewald, P. P. & Höln, H. (1936a). Ann. Phys., Lpz.[5], 25, 281
Ewald, P. P. & Höln, H. (1936b). Ann. Phys., Lpz.[5], 26, 173
Guillot, B., Jelsch, C., Muzet, N., Lecomte, C., Howard, E., Chevrier, B., Mitschler, A., Podjarny, A., Cousson, A., Sanishvili, R. & Joachimiak, A. (2000). Acta Cryst., A56 (Supplement), s199
Guillot, B., Viry, L., Guillot, R., Lecomte, C. & Jelsch, C. (2001). J. Appl. Cryst., 34, 214-223
Hansen, N. K. & Coppens, P. (1978). Acta. Cryst., A34, 909-921
Hellner, E. (1977). Acta Cryst., B33, 3813-3816
Hirshfeld, F. L. (1976). Acta Cryst., A32, 239-244
Housset, D., Habersetzer-Rochat, C., Astier, J.-P. & Fontecilla-Camps, J. C. (1994). J.Mol.Biol., 238, 88-103
Housset, D., Benabicha, F., Pichon-Pesme, V., Jelsch, C., Maierhofer, A., David, S., Fontecilla-Camps, J. C. & Lecomte, C. (2000). Acta Cryst., D56, 151-160
Jelsch, C., Pichon-Pesme, V., Lecomte, C. & Aubry, A. (1998). Acta Cryst. D54, 1306-1318
Jelsch, C., Teeter, M. M., Lamzin, V., Pichon-Pesme, V., Blessing, R. H. & Lecomte, C. (2000). PNAS, 97, no. 7, 3171-3176
Lamour, V., Barth, P., Rogniaux, H., Poterszman, A., Howard, E., Mitschler, A., Van Dorsselaer, A., Podjarny, A. & Moras, D. (1999). Acta Cryst. D55, 721-723
Lamzin, V., Morris, R.J., Dauter, Z., Wilson, K.S. & Teeter, M. M. (1999). J.Biol.Chem., 274, 20753-20755.
Longhi, S., Czjzek, M. & Cambillau, C. (1998). Curr. Opin. Struct. Biol., 8, 730-737
Pichon-Pesme, V., Lecomte, C. & Lachekar, H. (1995). J. Phys. Chem., 99, 6242-6250.
Pietsch, U. (1981). Phys. Stat. Sol., (b) 103, 93
Pietsch, U. & Unger, K. (1981). Phys. Stat. Sol., (b) 104, 253
Pietsch, U. (1985). Phys. Stat. Sol., (a) 87, 151
Pietsch, U., Tsirelson, V. G. & Ozerov, R. P. (1986). Phys. Stat. Sol., (b) 138, 47-52
Sanchez-Portal, D., Ordejon, P., Artacho, E. & Soler, J.M. (1997). Int. J. Quant. Chem., 65, 453.
Scheringer, C. (1980). Acta Cryst., A36, 205-210
Sheldrick, G. M. (1990). Acta Cryst., A46, 467-473
Sheldrick, G. M. & Schneider, T. R. (1997). Methods in Enzymology, 277, 319-343
Stewart, R.F. (1969). J. Chem. Phys., 51, 4569
Wiest, R., Pichon-Pesme, V., Bénard, M. & Lecomte, C. (1994). J. Phys. Chem., 98, 1351-1362
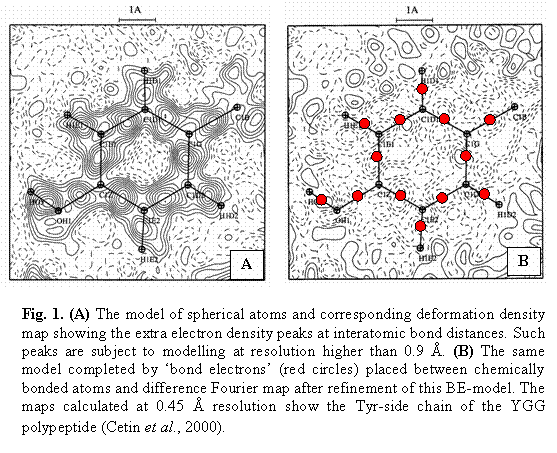
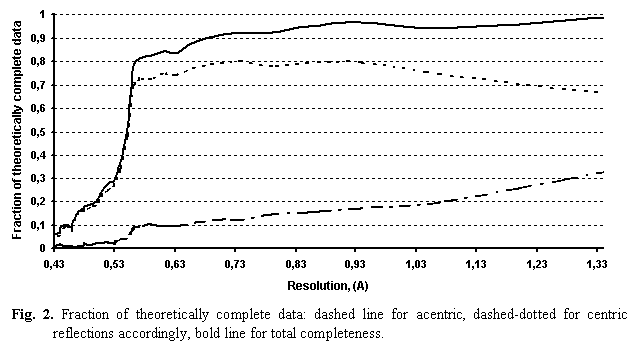
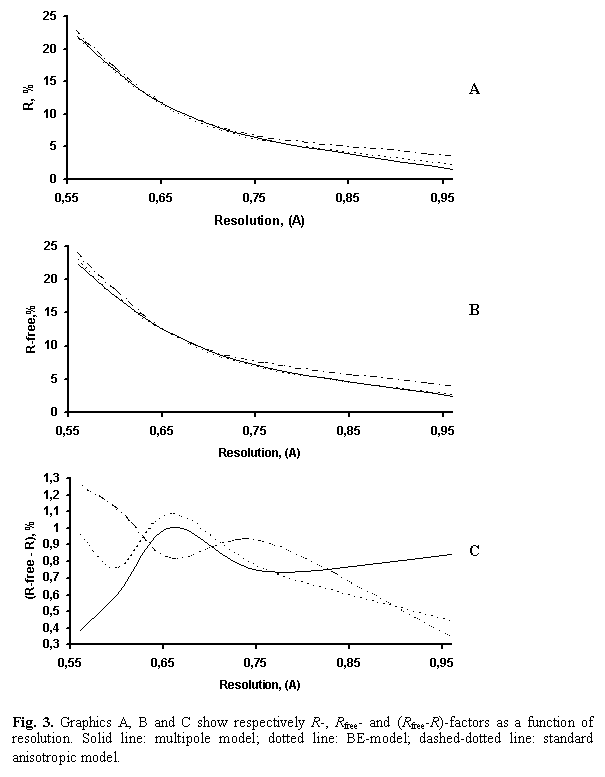
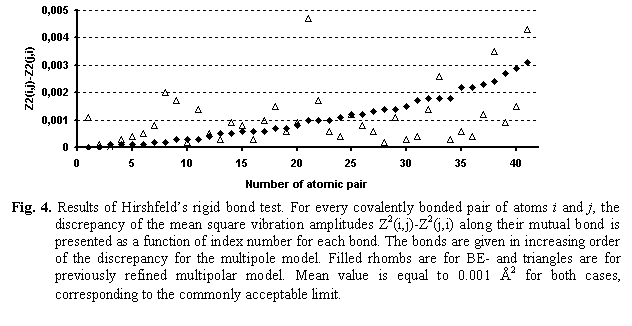
Table 1. Retrieved R / Rfree-factor
statistics for previously
refined multipolar model of enkephalin. M1 is the initial multipolar model
(Wiest et al., 1994), and M2 is the same model with recovered Rfree
statistics (see text, section 3, for details). Statistics is given for all
reflections with I > 0 . Models (resolution 0.56 -11.0 Å) R (F), % Rfree (F), % (Rfree - R),
% Number of reflections work set test set total M1 8.09 - - 8707 - 8707 M2 7.90 8.63 0.73 6894 1813 8707
Table 2. Refinement
statistics for different models Parameters *) R (I>0), % and data /
parameters Rfree (I>0), % (Rfree
- R), % Uaniso Biso Q XYZ Model A1 ® non-H atoms of
the model ® H-atoms of the model + - - + + fixed =1.0 + - 9.08 6894 / 474 9.74 0.66 Model BE1 ® non-H atoms of
the model ® H-atoms of the
model + - - - + + + fixed =1.0 + + - - 8.43 6894 / 658 9.16 0.73 Model BE2 ® non-H atoms of
the model ® H-atoms of the
model ® BE (bond
electrons) taken from ab initio calculations + - - - + + + fixed =1.0 + + - - 8.12 6894 / 658 8.76 0.64 *) 1) Uaniso
and Biso – anisotropic and isotropic displacement
parameters, respectively; Q – occupancy coefficient; XYZ – atomic coordinates 2) The sign ‘+’ or ‘-’ means that the
corresponding parameter was refined or fixed, respectively
Table
3.
Selected examples of parameters for bond critical points obtained from
BE2-model (Table 2) Atomic pair, A-B RA (Å) RB (Å) r (eÅ-3) l1 (eÅ-5) l2(eÅ-5) l3(eÅ-5) Ñ2(eÅ-5) h C1-C2 0.774 0.762 1.60 -8.8 -8.6 5.7 -11.7 1.54 C1-N1 0.621 0.881 1.59 -8.9 -8.8 12.3 -5.5 0.73 C25-C26 0.775 0.747 1.56 -8.9 -8.8 5.2 -12.5 1.72 C23-C24 0.781 0.756 1.53 -9.7 -9.5 4.5 -14.2 2.14 C24-C25 0.768 0.757 1.53 -8.4 -7.9 5.8 -10.5 1.44 R – distance from the critical point to
the neighbouring atom r – value of
electron density at the critical point l1, l2, l3 – eigenvalues of the normal matrix of the electron density
calculated in this critical point (the matrix of second derivatives with
respect to three coordinates) Ñ2 – Laplacian h = |l1| / l3
Model composition
® BE (bond electrons) taken as
H-atoms